In May, the World Health Organization (World Health Organization) issued an alarming report that widely used sugar-free sweeteners such as aspartame.
The WHO has reported that they are probably ineffective for weight loss and that long-term consumption may increase the risk of diabetes, cardiovascular disease and mortality in adults. A few months later, the WHO declared aspartame, the main ingredient in Diet Coke, a "probable carcinogen," but then quickly issued a third report that appeared to contradict its earlier findings. The third report stated that we can continue to consume the product at levels that were set safe πριν από δεκαετίες, πριν η νέα επιστημονική έρευνα εγείρει ανησυχίες για την health.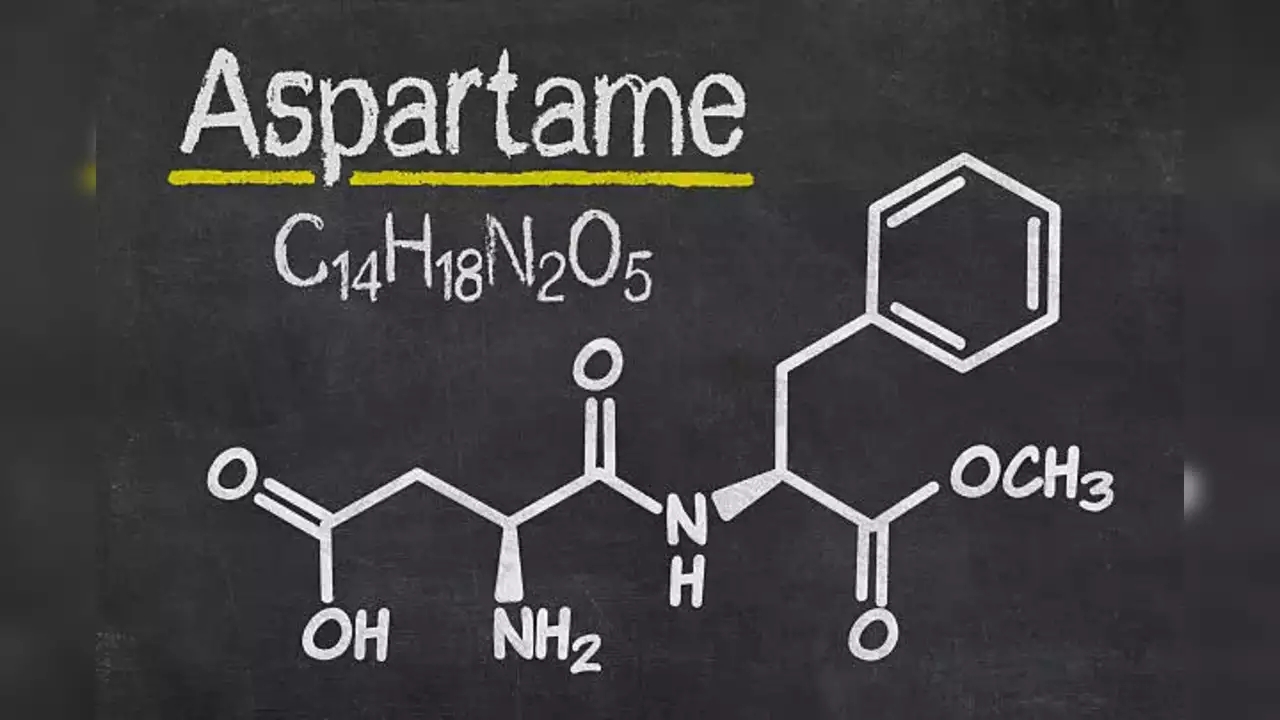
This contradiction comes from corruption of the review process by consultants connected to a Coca-Cola group;, the public health advocacy group said in a recent publication US Right-To-Know.
It revealed eight WHO panellists involved in assessing safe levels of aspartame consumption are consultants to the soft drink industry or have previously worked with a Coke scientific group, the International Life Sciences Institute (Ilsi).
Their involvement in agencies that create or approve guidelines represents “an obvious conflict interests," said Gary Ruskin, executive director of Right-To-Know USA.
"Because of this conflict of interest, the conclusions [on daily intake] for aspartame are not reliable and the public should not rely on them."
Ilsi describes itself as a non-profit organization that conducts "science for the public good," but was founded in 1978 by a Coca-Cola executive who worked at the company until 2021, according to Right-To-Know's USA. Other Coca-Cola executives have worked for the group, and their detailed tax returns show millions in donations from Coca-Cola and other drinks industry players. Coke ended its official membership of the group in 2021.
Over the years, Ilsi representatives have tried to shape policy for the τρόφιμα worldwide and Ruskin, who has written several scientific papers for the group, called the aspartame controversy "a masterpiece of how Ilsi gets into these regulatory processes."