The children they will always find ways not to go to school and the last one is tricking the COVID-19 lateral flow tests (LFT) to show positive results using soft drinks. So how can fruit juices and coke fool the tests?
If you do a simple test and drop a drop of orange soda or coke directly into an LFT, a few minutes later they will appear two lines on the test, indicating the presumed presence of the virus that causes COVID-19.
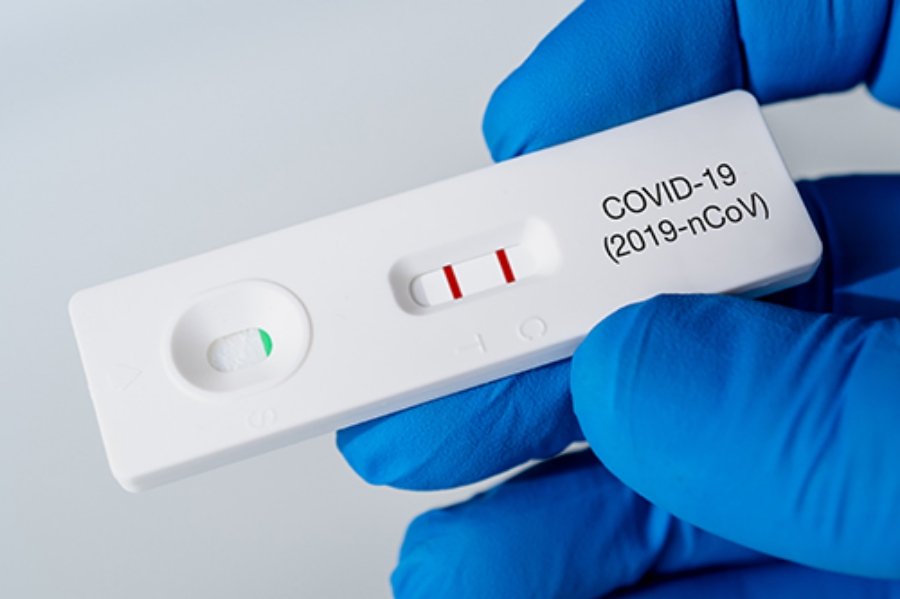
But let's see how the tests work. If you open an LFT device, you will find a paper-like thing called nitrocellulose and a small red pad, hidden under the plastic casing. The absorbent red pad contains the antibodies associated with the COVID-19 virus. There are also gold nanoparticles (tiny gold particles that later appear as red), which allow us to see where the antibodies are in the device. When you do a test, you mix your sample with a liquid buffer, ensuring the sample has the optimal pH, before instilling it into the device.
The fluid is absorbed by the nitrocellulose strip and "rests" on the gold and antibodies. The positive son sticks me to the gold of the antibodies and goes through a second set of antibodies. The virus then binds to both antibody sets by displaying a double line in the test, effectively indicating that whoever did the test is positive for the coronavirus.
So how can a soft drink cause a red line to appear?
One possibility is that the drinks contain something that the test recognizes as a virus. But this is rather unlikely. The samples collected are by nose or mouth, and seem to be completely unaware of a mess of proteins, other viruses and leftovers from your breakfast. Therefore, they are not going to react to the ingredients of a soft drink.
Another more likely explanation is that something in the drinks affects the function of the antibodies. All liquids that can fool the tests have one thing in common: they are acidic. Citric acid in orange juice, phosphoric acid in Coca-Cola and malic acid in apple juice give these drinks a pH between 2,5 and 4. These are very harsh conditions for antibodies, which work with almost neutral pH around 7,4.
Maintaining an ideal pH for antibodies is the key to the proper functioning of the test and this is the job of the solution (liquid buffer) with which you mix your sample. The crucial role of the solution is shown by the fact that if you mix it with Coca-Cola then the LFT will behave exactly as one would expect: the test will be negative.
Thus, without the buffer, the test antibodies are exposed to the acidic pH of the beverages. This seems to have a dramatic effect on their structure and function. Antibodies are proteins, which are made up of amino acid building blocks, joined together to form long, linear chains. Even a small change in these chains can dramatically affect the function of a protein.
Thus in acidic conditions, the protein is charged more and more positively. As a result, many of the interactions that hold the protein together are disrupted, its fine structure is affected, and it no longer functions properly. In this case, the sensitivity of the antibodies to the virus is lost.